What Does the IRB Review?, Research
Por um escritor misterioso
Descrição
Below are the elements the IRB looks for when reviewing research. Federal regulations 45 CFR 46.111 and 21 CFR 56.111 outline the requirements for approval of non-exempt human subjects research. To obtain IRB approval, the IRB must have enough information to determine the criteria in each of the sections below are satisfied.

Activities Requiring IRB Review - Office of Research Support and Compliance
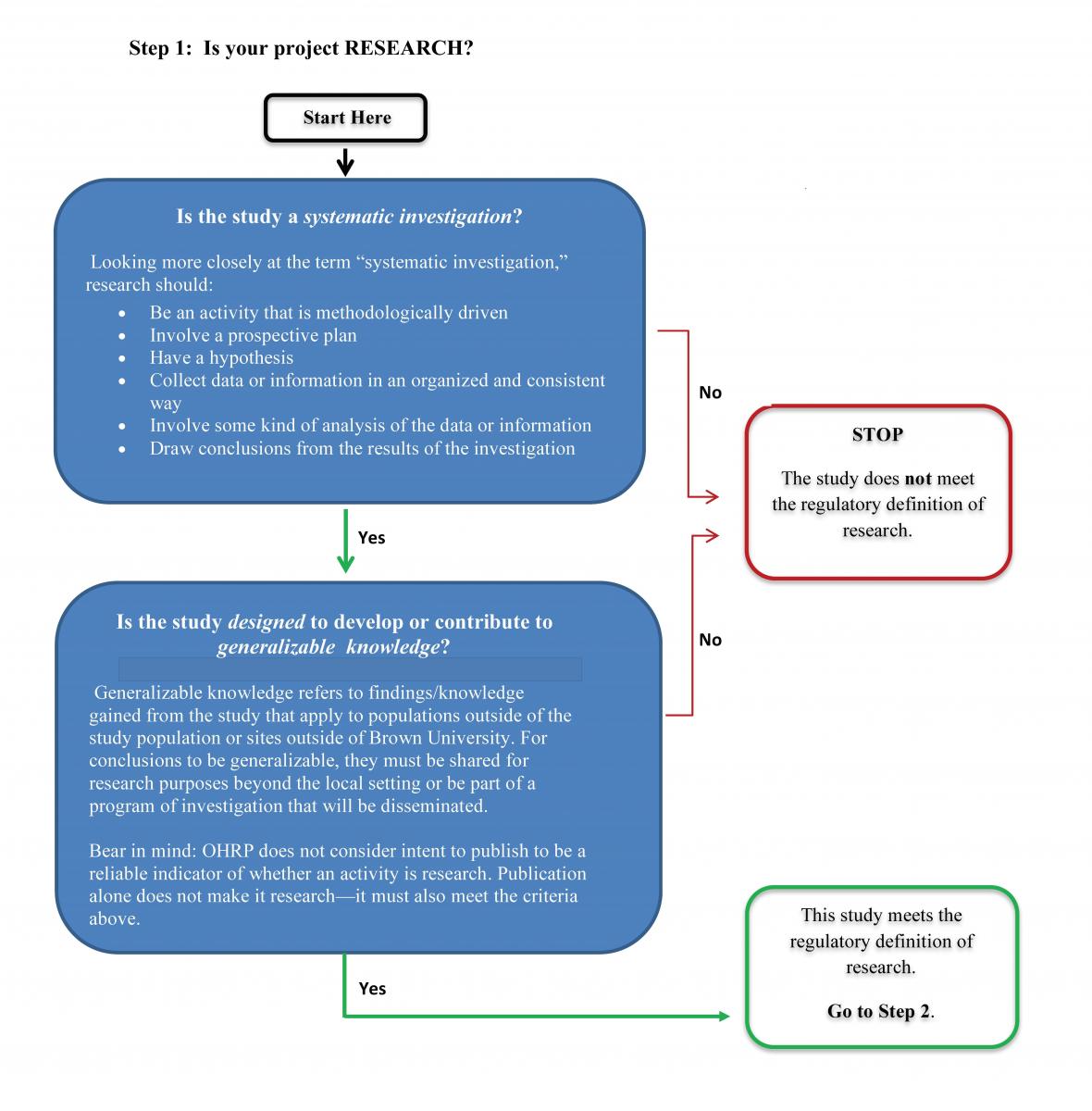
Does My Project Need IRB Review?, Research at Brown
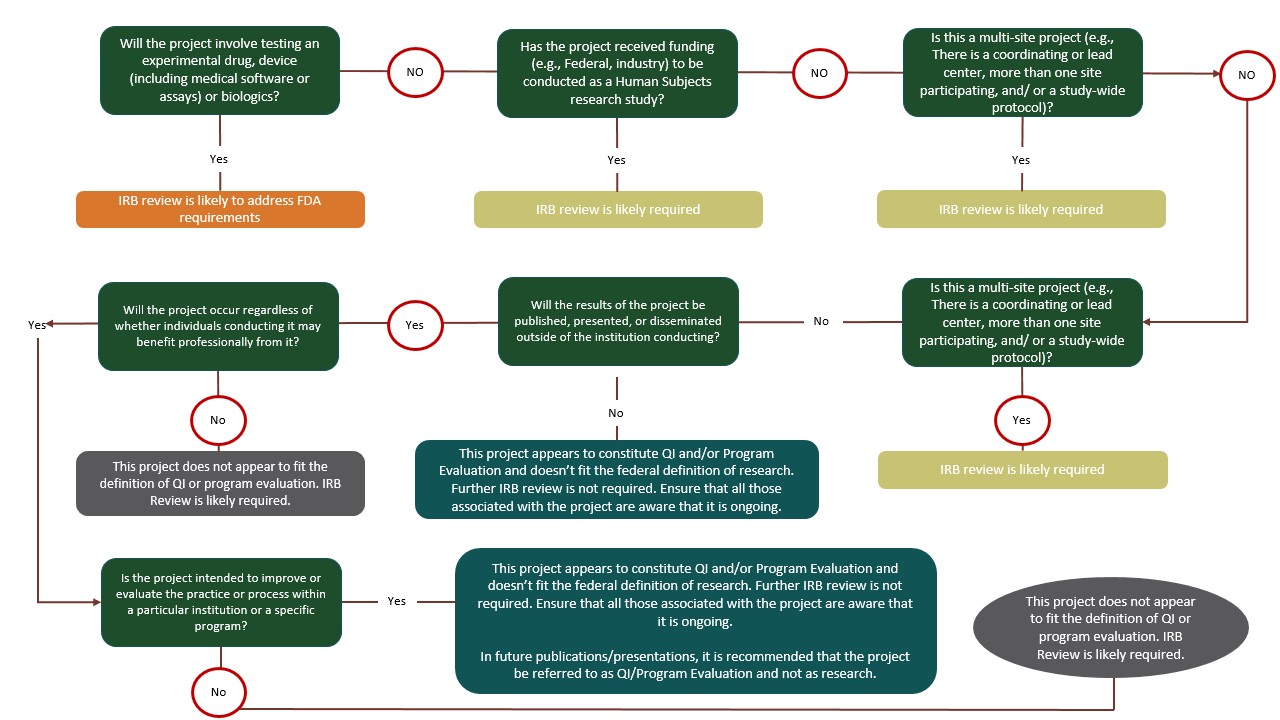
FAQs - Vice President For Research
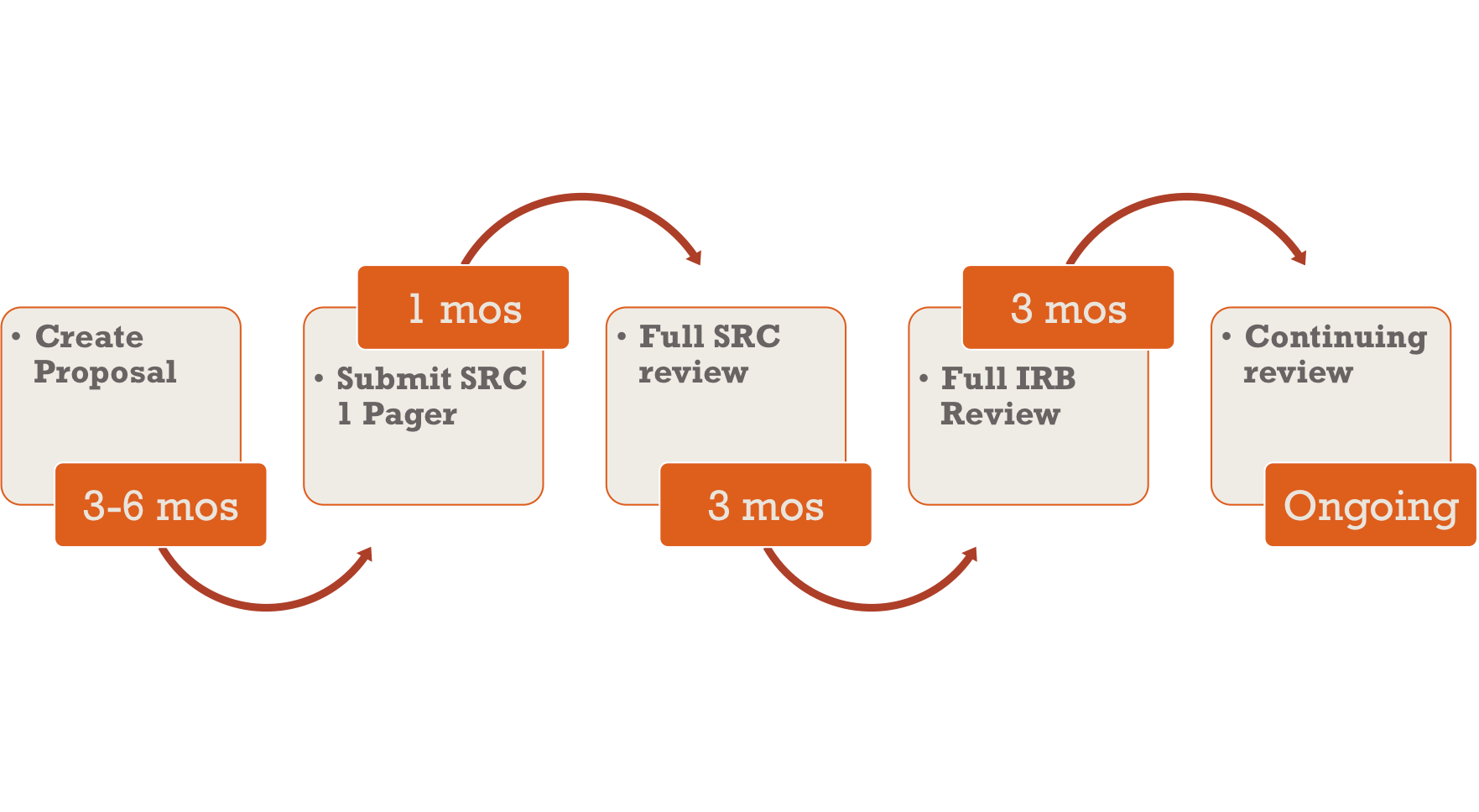
Scientific Review and IRB Submissions - National University of Natural Medicine
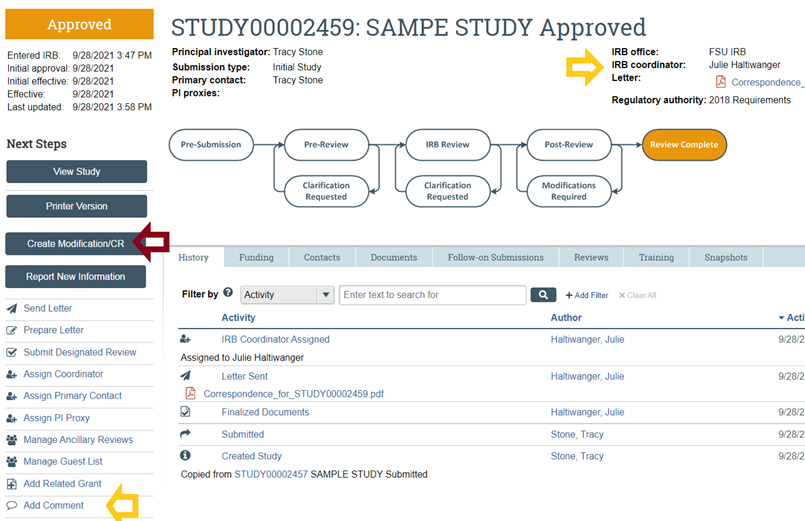
FAQs FSU Office of Research

Activities Requiring IRB Review - Office of Research Support and Compliance
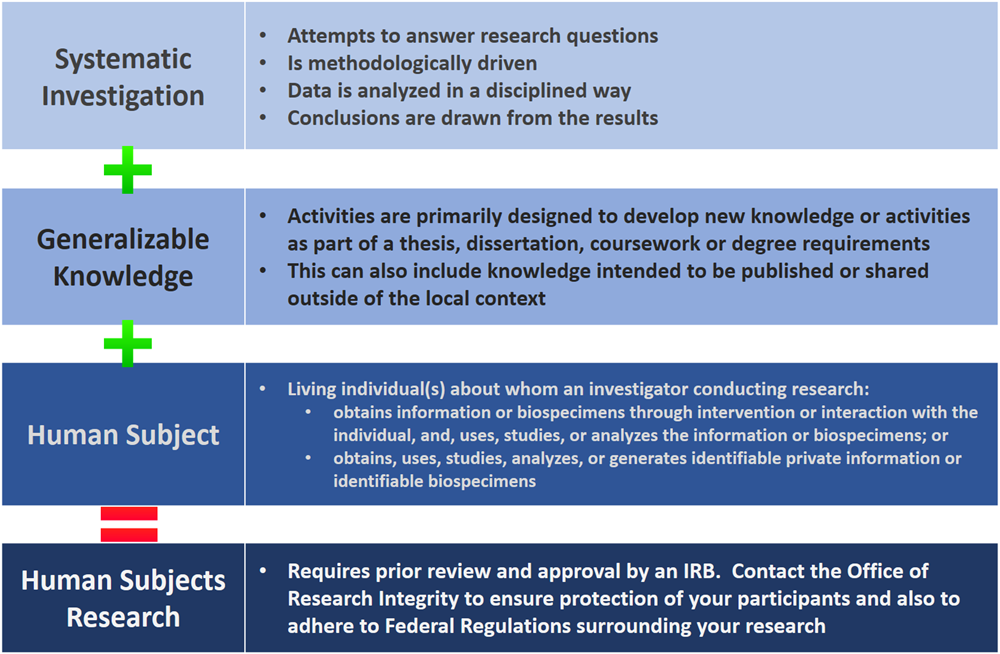
Frequently Asked Questions - Institutional Review Board (IRB)
Doctoral Dissertation Research and the IRB, 2021, IRB Blog, Institutional Review Board
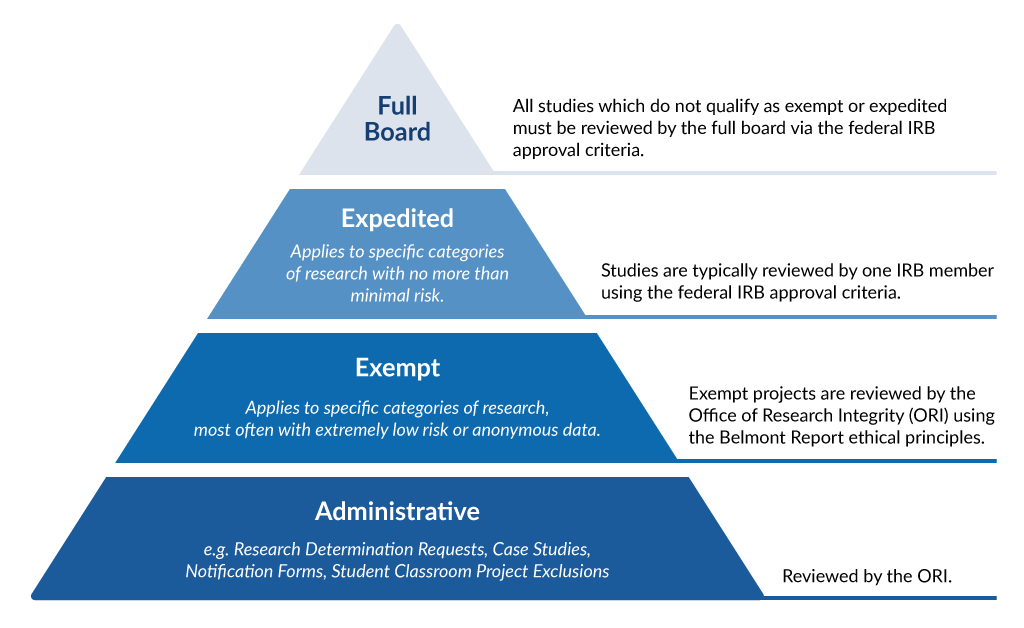
Frequently Asked Questions University of New England in Maine
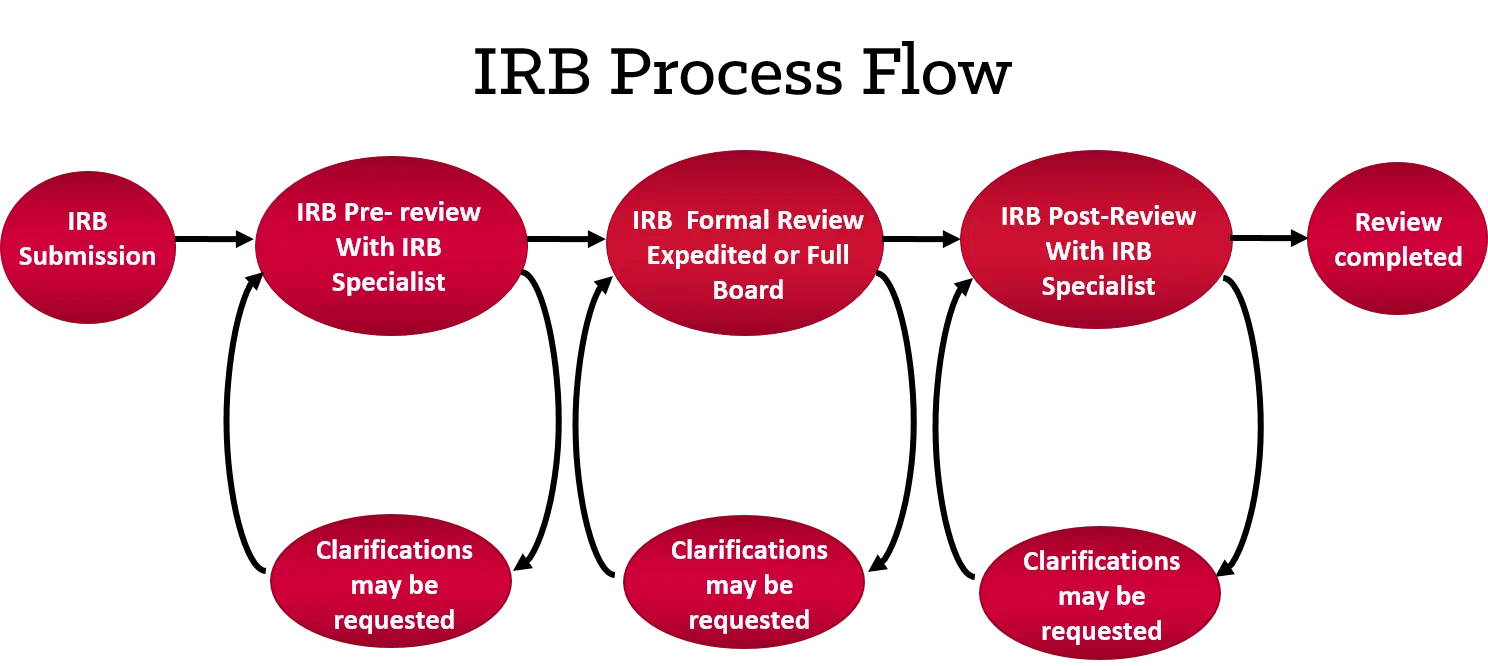
Institutional Review Board, Human Research Protection Program, University Hospitals, Cleveland, OH
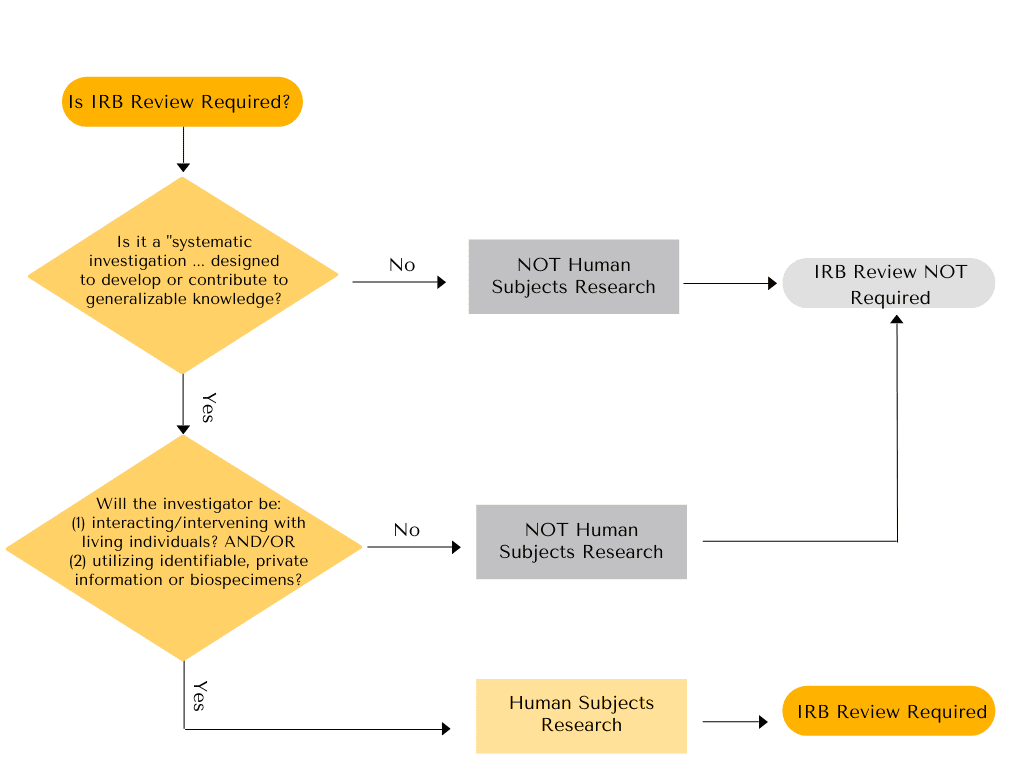
Deep Dives: What is the Difference Between “Exempt” Human Subjects Research, and Projects that are Not Human Subjects Research (NHSR)? – VCU Human Research Protection Program (HRPP) Blog

IRB reliance: An informatics approach - ScienceDirect

IRB Performance & Metrics
de
por adulto (o preço varia de acordo com o tamanho do grupo)