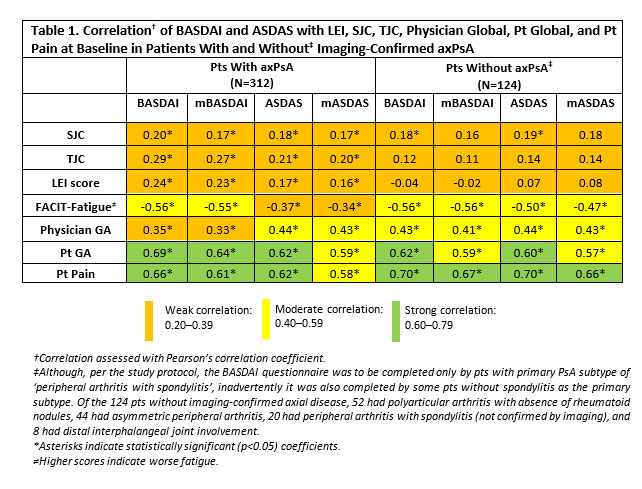
Subgroups according to BASDAI/ASDAS category (baseline)
Por um escritor misterioso
Descrição

Effects of ixekizumab treatment on structural changes in the sacroiliac joint: MRI assessments at 16 weeks in patients with non-radiographic axial spondyloarthritis - The Lancet Rheumatology
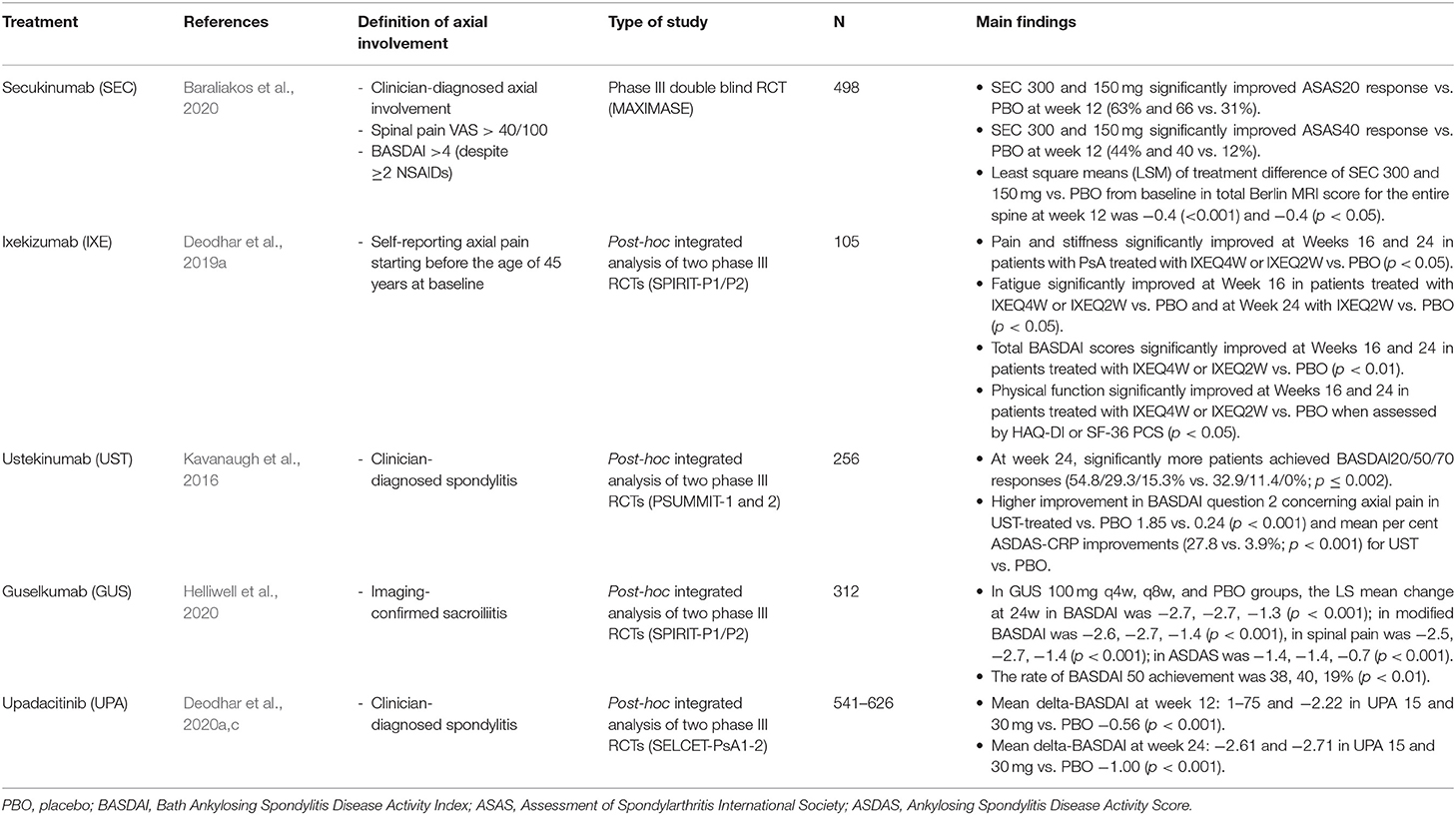
Frontiers Targeted Therapies in Axial Psoriatic Arthritis
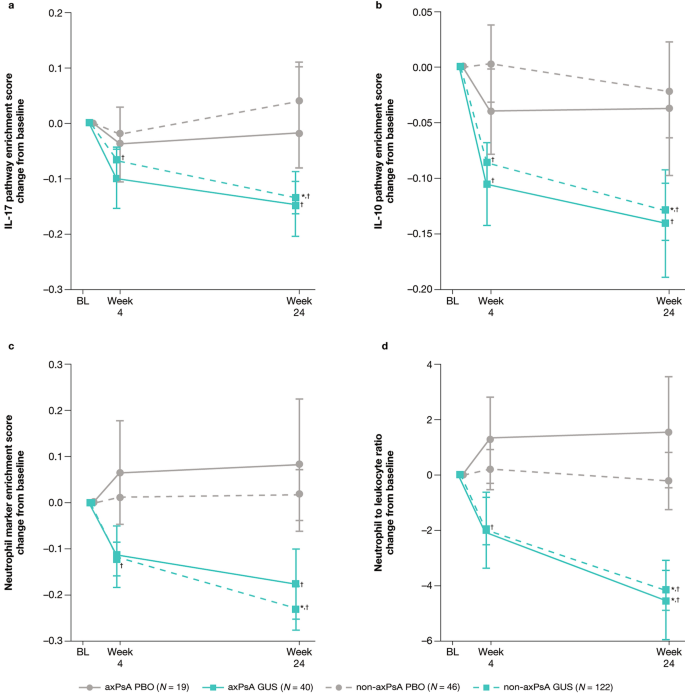
Genetic and Molecular Distinctions Between Axial Psoriatic Arthritis and Radiographic Axial Spondyloarthritis: Post Hoc Analyses from Four Phase 3 Clinical Trials

Standardized ASDAS vs standardized SASDAS for (A) baseline pooled data

(PDF) Both Baseline Clinical Factors and Genetic Polymorphisms Influence the Development of Severe Functional Status in Ankylosing Spondylitis

Frontiers Prediction of radiographic progression pattern in patients with ankylosing spondylitis using group-based trajectory modeling and decision trees
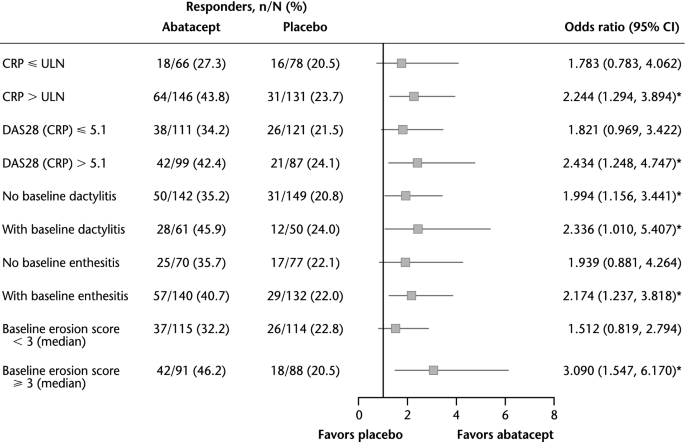
Poor prognostic factors in predicting abatacept response in a phase III randomized controlled trial in psoriatic arthritis
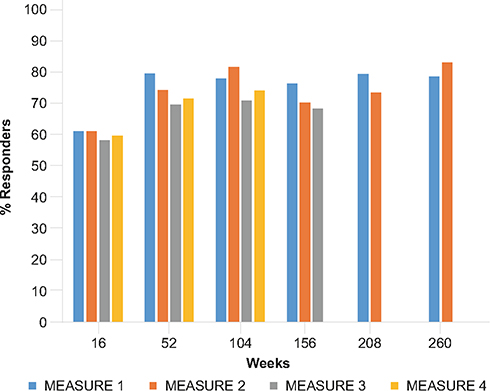
Impact of Secukinumab on Patient-Reported Outcomes in the Treatment of

A) Ankylosing Spondylitis Disease Activity Score (ASDAS) clinically
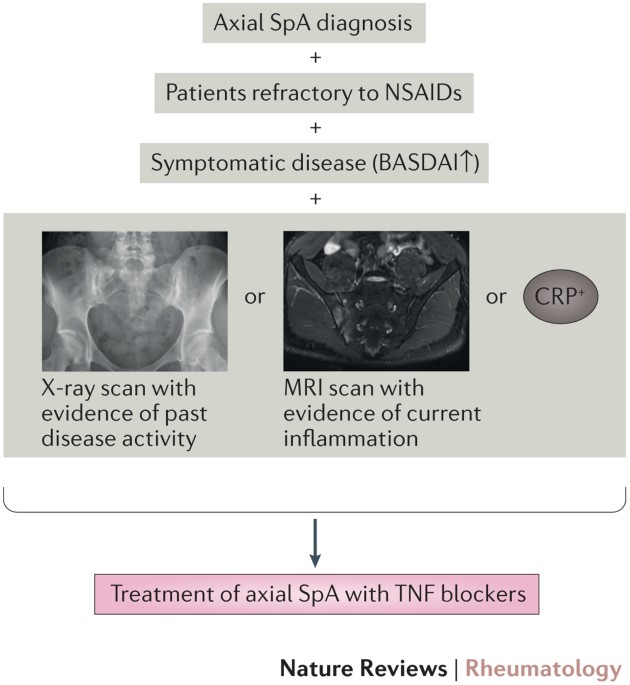
New evidence on the management of spondyloarthritis

Subgroups according to BASDAI/ASDAS category (baseline)

Full article: POSTER PRESENTATIONS
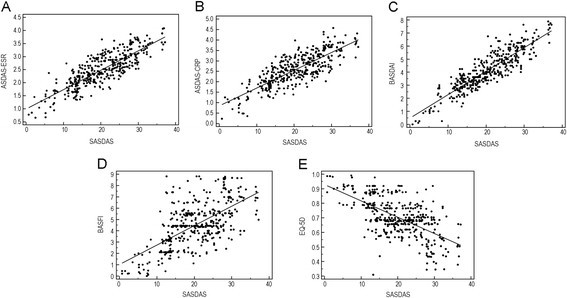
Construct validity and responsiveness of the simplified version of Ankylosing Spondylitis Disease Activity Score (SASDAS) for the evaluation of disease activity in axial spondyloarthritis, Health and Quality of Life Outcomes

Secukinumab provided significant and sustained improvement in the signs and symptoms of ankylosing spondylitis: results from the 52-week, Phase III China-centric study, MEASURE 5
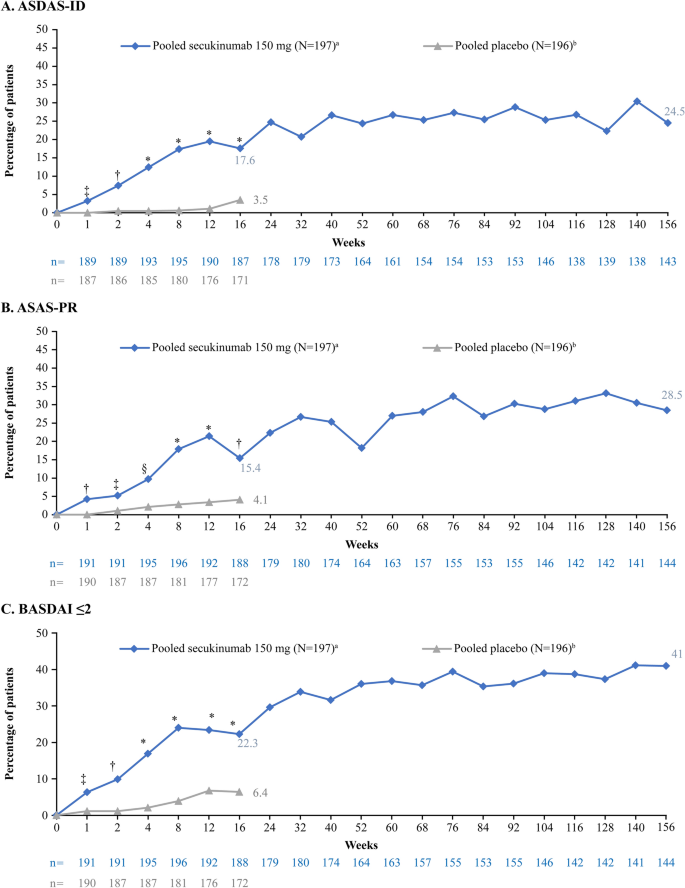
Achievement of Remission Endpoints with Secukinumab Over 3 Years in Active Ankylosing Spondylitis: Pooled Analysis of Two Phase 3 Studies
de
por adulto (o preço varia de acordo com o tamanho do grupo)