Statistics in Medicine — Reporting of Subgroup Analyses in
Por um escritor misterioso
Descrição

The lack of statistical power of subgroup analyses in meta-analyses: a cautionary note, Epidemiology and Psychiatric Sciences

Table 1 from How to use a subgroup analysis: users' guide to the medical literature.

PDF) Statistics in Medicine — Reporting of Subgroup Analyses in Clinical Trials
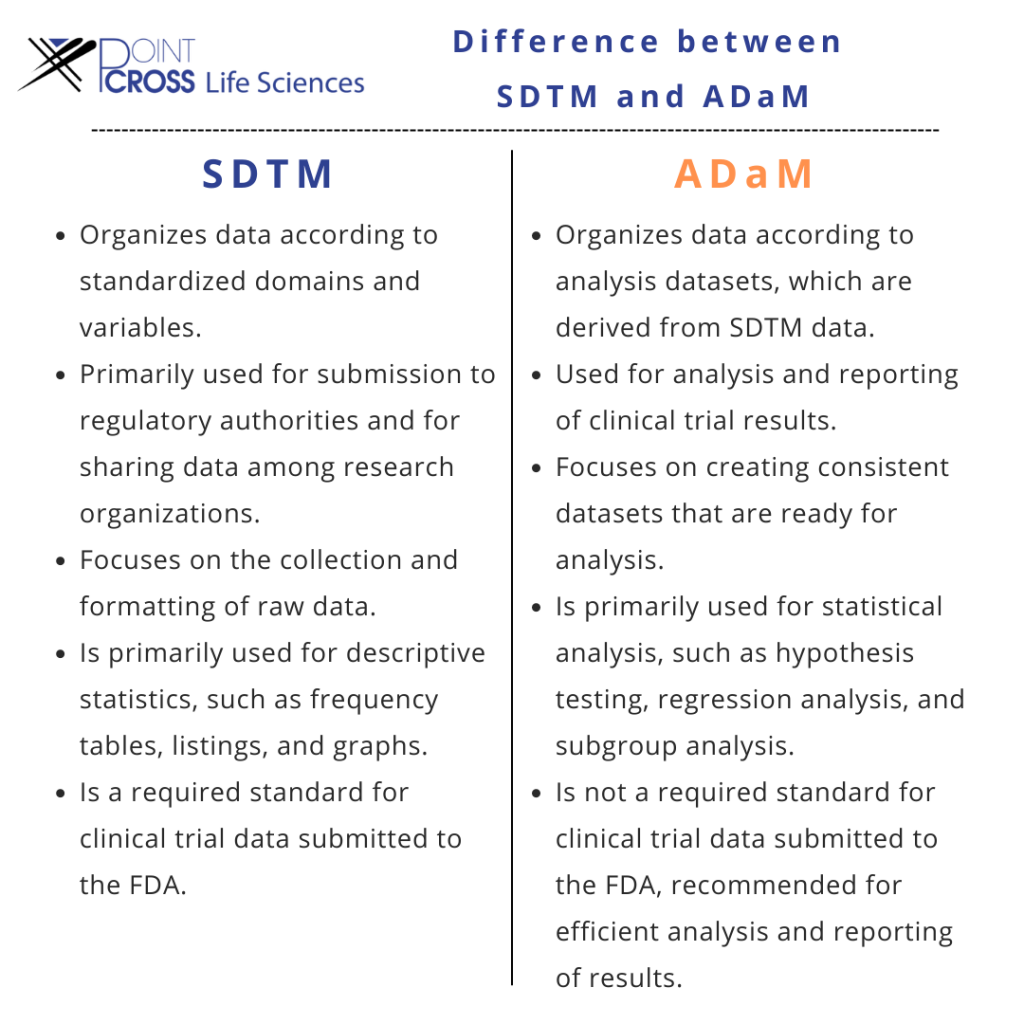
SDTM - Everything that you need to know

Understanding of interaction (subgroup) analysis in clinical trials - Brankovic - 2019 - European Journal of Clinical Investigation - Wiley Online Library

Subgroup analysis in randomised controlled trials: importance, indications, and interpretation - ScienceDirect
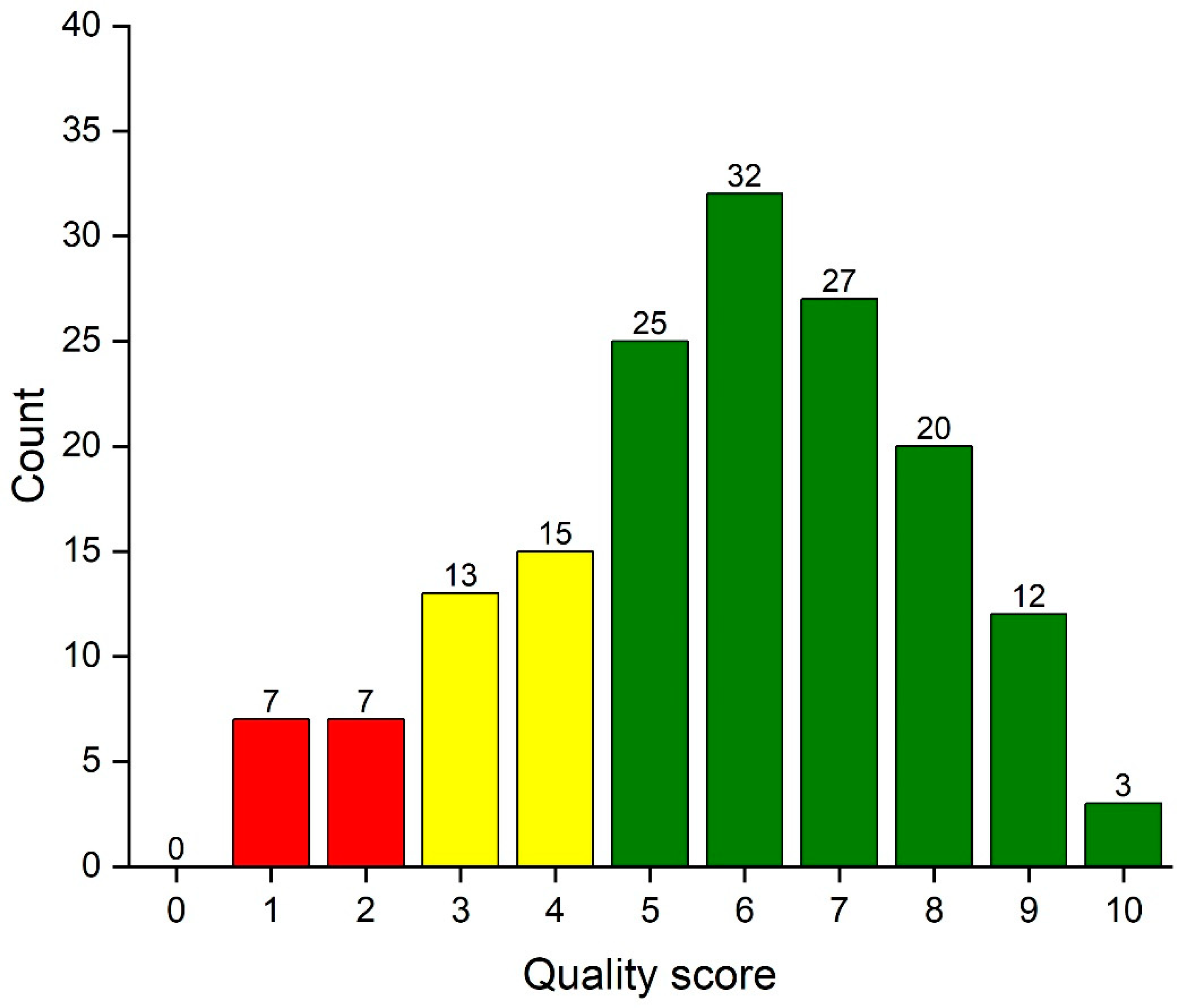
Applied Sciences, Free Full-Text

Statistics in Medicine — Reporting of Subgroup Analyses in Clinical Trials

Full article: Tezepelumab compared with other biologics for the treatment of severe asthma: a systematic review and indirect treatment comparison

Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data - The Lancet Diabetes & Endocrinology
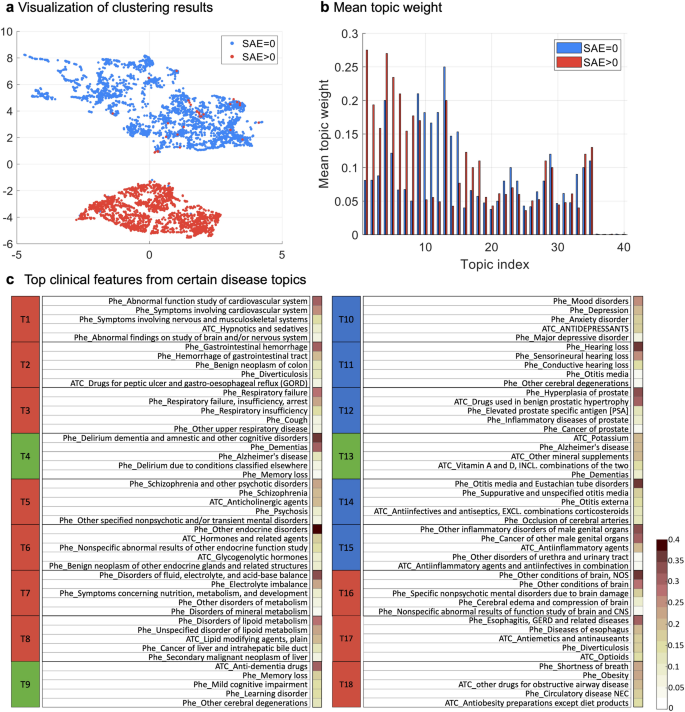
Machine learning enabled subgroup analysis with real-world data to inform clinical trial eligibility criteria design

A critical review of graphics for subgroup analyses in clinical trials - Ballarini - 2020 - Pharmaceutical Statistics - Wiley Online Library

Figure 2 from How to use a subgroup analysis: users' guide to the medical literature.

School Connectedness and Risk Behaviors and Experiences Among High School Students — Youth Risk Behavior Survey, United States, 2021
de
por adulto (o preço varia de acordo com o tamanho do grupo)