Hep B biotech Antios closed after FDA hold proved insurmountable
Por um escritor misterioso
Descrição
Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable. | Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable.

Landon Loving on LinkedIn: Fierce Biotech Fundraising Tracker '23
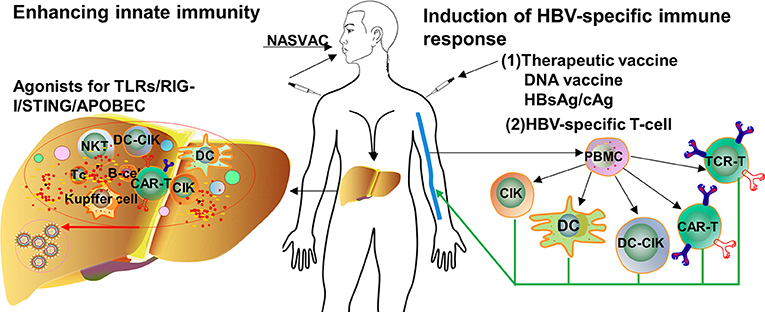
Frontiers Advances in Targeting the Innate and Adaptive Immune

Heart trouble report & clinical hold spell end of Antios, Assembly

Landon Loving sur LinkedIn : GSK's $100M ADC bet in doubt after

Hepatitis B Foundation

Heplisav-B: A New Hepatitis B Vaccine That Can Be Used For Pre
Biotechs jockey for gene therapy lead with hemophilia data

IHEP (International Hepatology Education Program)

Biotech Fierce Biotech

Bone Scaffolds: An Incorporation of Biomaterials, Cells, and

IHEP (International Hepatology Education Program)

Hepatitis B drug developers chart slow progress, just like in hep C
de
por adulto (o preço varia de acordo com o tamanho do grupo)