ASAS-HI improvement ≥30%, ASDAS LDA status and ASAS40 response
Por um escritor misterioso
Descrição

Efficacy of a tight-control and treat-to-target strategy in axial spondyloarthritis: results of the open-label, pragmatic, cluster-randomised TICOSPA trial. - Abstract - Europe PMC
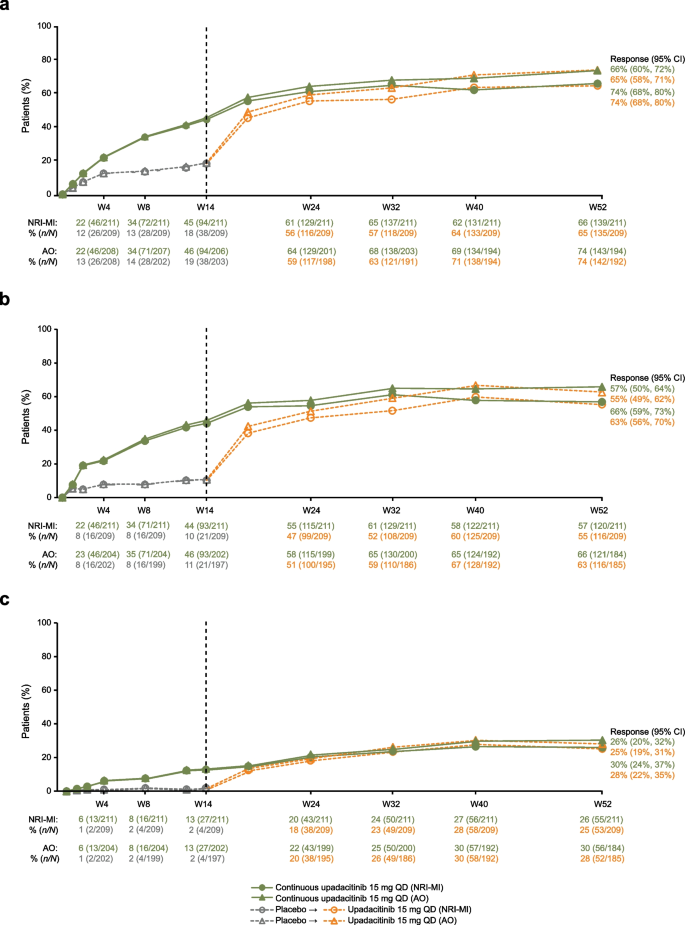
Efficacy and safety of upadacitinib in patients with ankylosing spondylitis refractory to biologic therapy: 1-year results from the open-label extension of a phase III study, Arthritis Research & Therapy

UCB Presents New Five-Year Data on BIMZELX® (bimekizumab-bkzx) in Ankylosing Spondylitis at ACR Convergence 2023

PDF] ASAS40 and ASDAS clinical responses in the ABILITY-1 clinical trial translate to meaningful improvements in physical function, health-related quality of life and work productivity in patients with non-radiographic axial spondyloarthritis
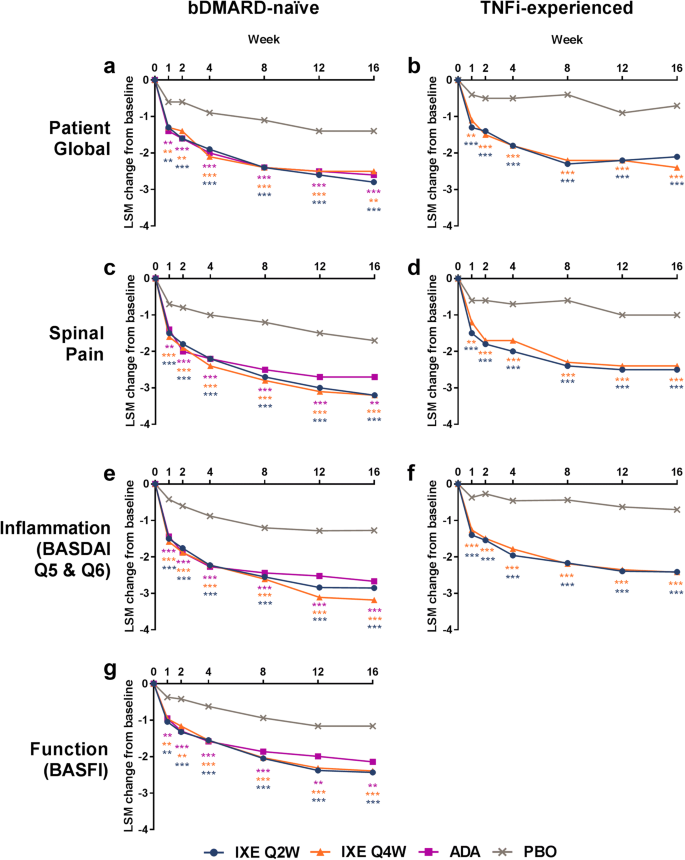
Translating Improvements with Ixekizumab in Clinical Trial Outcomes into Clinical Practice: ASAS40, Pain, Fatigue, and Sleep in Ankylosing Spondylitis
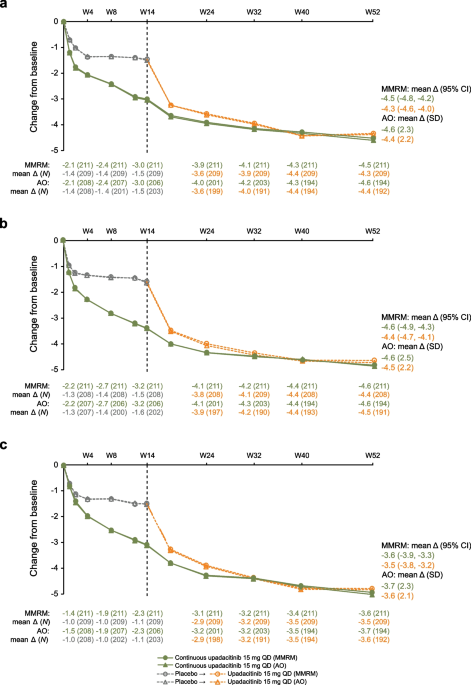
Efficacy and safety of upadacitinib in patients with ankylosing spondylitis refractory to biologic therapy: 1-year results from the open-label extension of a phase III study, Arthritis Research & Therapy
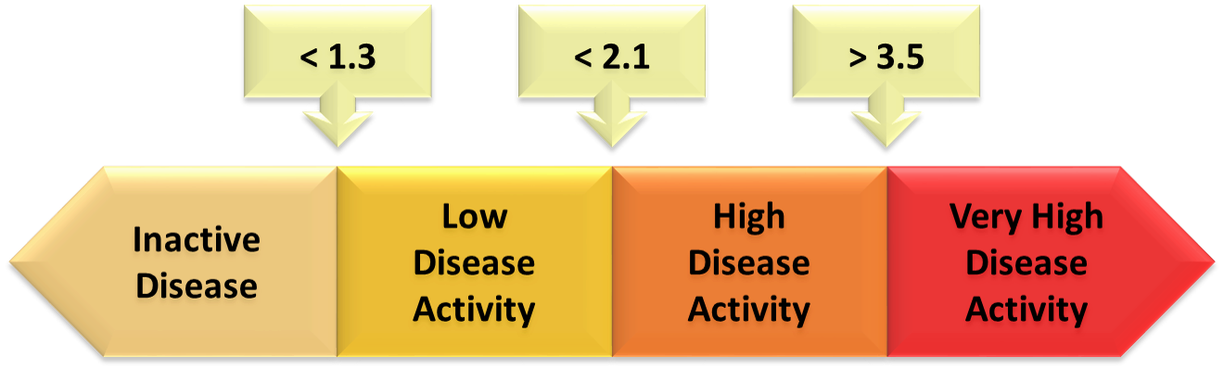
ASDAS calculator - ASAS

AS Criteria: Diagnosis (ASAS), Disease Activity (ASDAS), Radiographic Progression (mSASSS) - Arthritis Rheumatism
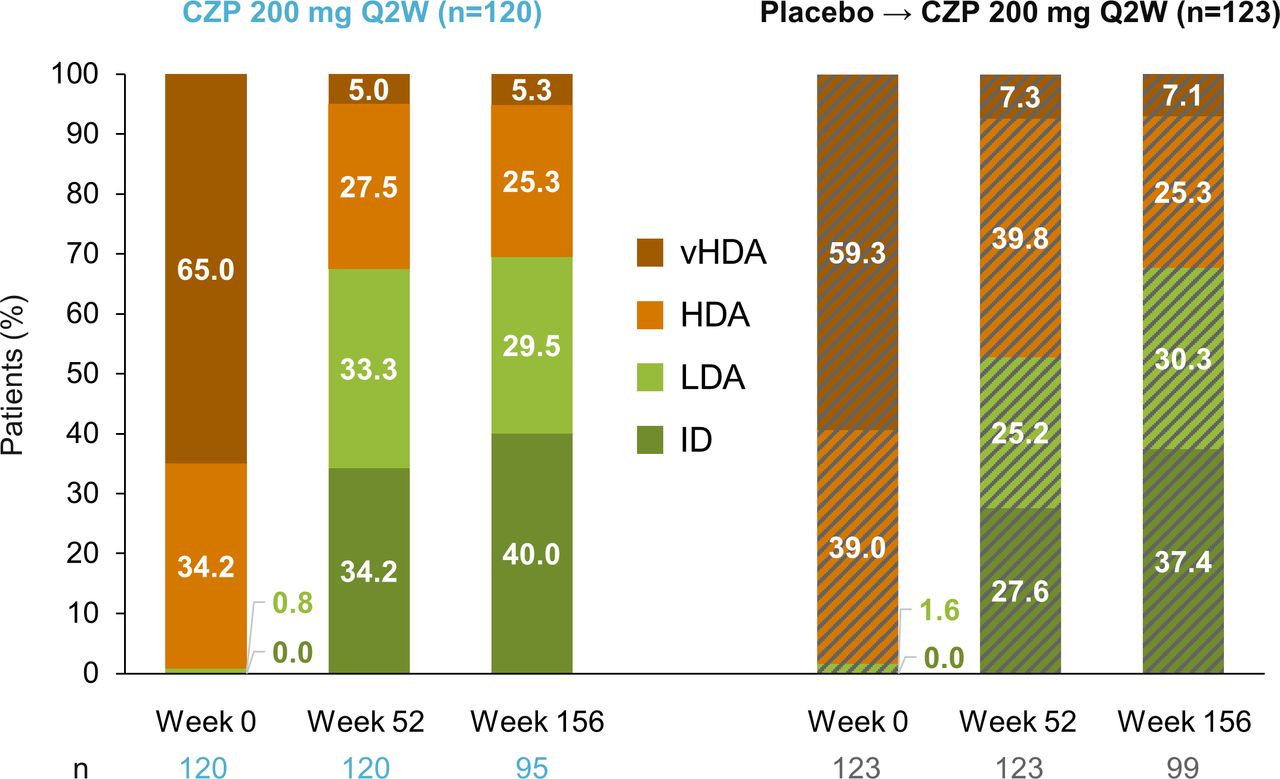
Long-term safety and clinical outcomes of certolizumab pegol treatment in patients with active non-radiographic axial spondyloarthritis: 3-year results from the phase 3 C-axSpAnd study

Disease Control Data, Ankylosing Spondylitis
de
por adulto (o preço varia de acordo com o tamanho do grupo)